These are the Papers I and II from the Patent Agent Examination 2013 conducted on 4th May 2013
Refer to the official website for the latest syllabus and updates.
Instructions for PAPER I
1. This paper consists of 2 parts—Part A (40 marks) & Part B (60 marks).
2. ALL questions in Part A are compulsory.
3. Part A comprises two sections – Part A1 and A2. Part A1 is of 30 marks and consists of 15 multiple choice questions of 2 marks each. Part A2 is of 10 marks and consists of 10 true/false type questions of 1 mark each.
4. Part B consists of 8 questions out of which the candidates should answer 6 questions (10 marks each). In case a candidate answers more than 6 questions, the first 6 questions will be evaluated.
5. Candidates should read the questions very carefully before answering.
6. No clarification will be provided during the course of the examination.
7. There is no negative marking.
8. Max. Marks: 100
9. TIME: Three Hours
PART A
PART A1
15 x 2 = 30 marks
1. The statement and undertaking regarding the filing of foreign application by an applicant shall be filed
a. Within six months of the filing of such application
b. Immediately after grant of the foreign patent
c. Simultaneously on the date of foreign filing
d. Within one year of the fiing
2. A divisional application may be filed by the applicant
a. Within six months from the date of filing provisional application
b. Within 31 months of the filing of a conventional application
c. Any time before the grant of patent on the first mentioned application
d. Before the publication of the patent application
3. The appropriate court for institution of a suit for infrinqment of patent is
a. The District Court
b. The High Court
c. IPAB
d. (a) or (b)
4. In settling the terms and conditions of a compulsory license, the Controller shall endeavor to secure
a. That the licence granted is exclusive and non-assignable
b. That the licence is for a period of twenty years from the date of grant of licence
c. That the patented invention is worked to a reasonable extent
d. None of the above
5. Information relating to an unpublished patent application filed at the patent office can be obtained by any person
a. Immediately after filing the application
b. On payment of prescribed fees
c. By physical inspection of the file at the patent office
d. None of the above
6. When a patent is sought to be revoked on the ground of wrongful obtaining from the true and first inventor, the Controller may
a. Direct that the patent shall stand amended in the name of the opponent
b. Direct the person wrongfully obtaining to pay penalty to the true and first inventor
c. Revoke the patent and ask the rightful inventor to apply afresh.
d. Direct the central government to pay compensation to the inventor.
7. In which of the following cases, an appeal shall not lie to the IPAB?
a. Refusal of a patent application by the Controller under section 15
b. Decision regarding post-dating of an application under section 17
c. Decision of the Controller under section 54 regarding patent of addition
d. An application is deemed to have been abandoned under section 21
8. When an application for a patent is published but not vet granted, any person can oppose the patent application on the following grounds
a. Non-payment of prescribed fees
b. Non-disclosure of financial assets by the applicant before the patent office
c. The applicant has not provided an address of service
d. The invention is anticipated having regard to the knowledge of an indigeneous community in South Africa.
9. The Controller in any proceedings before him under the Patents Act, 1970 has the powers of a civil court while trying a suit under the Code of Civil Procedure. 1908 in respect of the following matters
a. passing an order regarding search and seizure of infringing products of an opponent who has also filed a post-grant opposition
b. requiring the discovery and production of any document
c. receiving evidence by way of declaration
d. passing an order granting interim injunction against alleged infringers
10. One of the following is not true in relation to a patent of addition
a. If the patent for the main invention is revoked, the patent of addition may continue as an independent patent for the remaining part of the term of the main patent
b. Renewal fee is to be paid to keep the patent of addition alive if the main patent is revoked
c. A patent of addition can be granted before the grant of the patent for the main invention
d. The term of the patent of addition is generally less than the term of the patent for the main invention
11. Identify the option in which both Ramesh and Suresh cannot be inventors as per the Indian Patents Act 1970
a. Ramesh and Suresh work for the identification of a novel process in the laboratory
b. Ramesh finances Suresh work for the identification of a novel process in the laboratory
c. Ramesh, from US and Suresh from India work on identification of a novel process in the laboratory
d. Ramesh conceptualizes the invention and joins Suresh in reducing the invention to practice
12. One of the following is not a ground for pre-grant and post-grant opposition of a patent in India
a. Wrongful obtaining of the invention
b. Non-disclosure or wrong mentioning of the source or geographical origin of biological material used for the invention
c. Complete specification does not sufficiently and clearly describe the invention
d. Invention was secretly used in India
13. A provisional patent specification must contain
a. Description of the invention
b. Claim or Claims defining the scope of Inventions
c. Abstract of the technical information
d. Background and prior art
14. In case of request for permission for making patent application outside India, the Controller shall dispose of the request within a period of
a. 15 days
b. 21 days
c. 30 days
d. None of the above
15. A post-grant opposition may be filed
a. By any person
b. Before the expiry of a period of 12 months from the date of publication under section 43
c. Before the expiry of a period of 12 months from the date of publication under section 11A
d. Before the expiry of 12 months from the date on which the Controller writes in the file ‘GRANTED’.
PART A2
Instructions:
1. Answer ALL questions. Each question carries one [l] mark.
2. Select whether the below propositions are “TRUE” or “FALSE”. You can only select either “TRUE” or “FALSE”. You must indicate your choice in the answer sheet given to you against the relevant question number.
3. Illustratively, if the right answer to Question (1) is “TRUE” you must write: Question (1) = TRUE.
10 x 1 = 10 Marks
1. Revocation of a patent on the ground of being mischievous to the State or prejudicial to the public can be done only by the High Court on application by any person interested.
2. If an applicant for a patent who has filed a provisional specification files a request for early publication before filing the complete specification, the application will be published within one month from the date of filing of such request.
3. It is mandatory to describe the closest prior art in a patent specification.
4. In order to get an assignment registered at the Patent Office, the application has to be filed for registration before the expiry of six months from the date of assignment.
5. An exclusive licensee of the Patent shall also have the right to sue for infringement of patents in any District Court adding the patentee as a defendant.
6. Use of the patented product for the purpose of development and submission of information before the Drugs Controller General of India is not considered as infringement.
7. A model or sample of anything illustrating the invention or alleged to constitute an invention, submitted by an applicant to supplement the application in compliance to a direction of the Controller shall be deemed to form part of the specification.
8. A patent may be revoked on a petition of any person interested or of the Central Government by the Appellate Board or on a counter-claim in a suit for infringement of the patent by the District Court on the grounds specified under the Act.
9. When secrecy directions are in force, Controller shall not pass an order refusing to grant the patent.
10. Priority of an application filed in a convention country cannot be claimed if the application filed in the convention country is withdrawn before the date of filing of the corresponding convention application in India.
PART B
This Part contains Eight [8] Questions. Answer any SIX [6] questions. Your answers should be supported by relevant provisions of the Patents Act and Patent Rules.
6 X 10 = 60 Marks
1. Rohit, the inventor of a locking mechanism for a refrigerator was invited by the Indian Institute of Science to present a paper in a conference. He presented the paper deliniating the invention in the abstract. The industrialists who attended Rohit’s presentation are now interested in commercial exploitation of the invention. Rohit has approached you for professional help in procuring patent protection for his invention. Advise him.
2. Sheela filed a patent application accompanied by a provisional specification on 1st January, 2013 for her invention relating to a carburettor which is novel and has an inventive step. In continuation of the research, she found a new process for manufacture of the carburettor and she filed another patent application accompanied by a provisional specification, three months after the date of filing of the first patent application. She wishes to file one complete specification in which she wishes to incorporate the subject matter disclosed in both the aforementioned applications, i.e. of the carburettor as a product and the process of manufacture. She has approached you for advice and assistance. Explain to her how you will proceed with the preparation of the documentation and ensure that all the formalities are taken care of as per the Indian Patents Act.
3. Salim holds a patent on an agricultural tool in India and in the US. Salim authorizes Bharat to produce and sell the patented tool in the US. Julie buys the agricultural tool from Bharat and imports it into India. Will Julie be liable for infringement in India? Explain.
4. A leading natural products specialist, Leelavati on her return to India set up a new institute called Herbal Advance Research Institute (HARI), in Lucknow. Researching in natural products, she filed a patent application in India, jointly with her colleague Dr. Prathiba for a herbal composition for water purification and called the product “PaniPure”. They were granted a patent for “PaniPure” as inventor applicants in 2010. Dr. Prathiba left HARI and joined a Water Treatment Company “AquaTreat” in Jammu to whom she licensed the patent. “AquaTreat” started marketing the product “PaniPure” based on this invention. Leelavati got to know about this development from the local newspapers. She has approached you for advice on the steps she can take to protect her interest. Please advise appropriately.
5. A patent applicant receives a First Examination Report for his application conveying the objections. Clearly explain all the possible options before the applicant while providing a brief overview of the process that may ensue.
6. What are the grounds to make an application for grant of compulsory licence on a patent under Section 84 (1)? What are the factors that the Controller is required to take into account while considering an application under this section.
7. Describe the powers of the Central Government to use inventions for the purpose of the Government along with the terms and conditions for such use.
8. What are the restrictive conditions that need to be avoided in the preparation of a contract or license related to patents and what are the consequences of such conditions in a contract or license as per the Patents Act?
PATENT AGENT EXAMINATION, 2013 – PAPER II
Exam held on 4th May 2013
Total Marks: 100 Time: 2.30 p.m. to 5.30 p.m. Three hours
Instructions:
1. This paper consists of 2 parts – Part A (40 marks) & Part B (60 marks).
2. ALL questions in Part A are compulsory.
3. Part A consists of 4 questions of 10 marks each.
4. Part B comprises two sections – Part B1 and B2, of 30 marks each. Part B1 consists of 2 questions and you are required to answer any 1 of them. Part B2 also consists of 2 questions and you are required to answer any 1 of them. In case a candidate answers more questions than required, the first attempted question will be evaluated.
5. Candidates should read the questions very carefully before answering.
6. No clarification will be provided during the course of the examination.
7. There is no negative marking.
PART A
4×10 =40 marks
1. Rupa, a textile designer with a ‘Design House’ in Mumbai having branch offices in various parts of the world, wishes to obtain patent protection in 50 different countries for smart textiles invented by her. Foreign buyers prefer to deal with inventor/applicants who have filed their patent applications in Europe as basic applications i.e. the first application to be filed on the subject matter. She also wishes to file a set of patent applications in Europe with varying priority dates. Rupa has approached you as her Patent Agent in India to help her in her mission. Provide a note to her outlining the strategy she ought to follow alongwith information on various processes / routes involved and the timelines involved. How would you also deal with the multiple priorities?
2. Korobi Sen in Kolkata has worked on the development of special sweet compositions for diabetic patients. These compositions also contain some herb extracts. The sweets developed by her have very special properties in that the blood sugar levels do not rise when these sweets are consumed. She has filed a patent application in the Patent Office, Kolkata claiming the compositions for her sweets and the method of making them. The Controller issued an examination report objecting to the grant of the patent by citing Sections 3(e), 3(i) and 3(p) of the Indian Patents Act. Korobi has approached you to respond to the first examination report (FER) and also to attend a hearing at the Patent Office in due course. Draft a response to the objections raised in the FER.
3. Anjan has been involved in the development of drainage systems. He thought it necessary to conduct tests in the compound of his large housing society to assess the scalability of his newly developed drainage system on 30th March 2009. On 1st September 2009, Anjan applied for a patent in the Mumbai Patent Office. Anjan was very confident that there was no prior art that could come in way of the grant of his patent application. Praful who lives in the same housing society and had seen the testing of the invention in their society, came to know about the patent application when it was published in the Journal of the Patent Office and opposed the patent application on the ground that he had witnessed the use of the invention in a public place in India before the patent application was filed. Please draft the statement in reply that is required to be submitted to the Patent Office.
4. A ship named ‘Voyager’ registered in Panama and operated by ‘White Waterlines’ from Brunei accidentally entered the territorial waters of India in the Bay of Bengal. The Indian Coast Guard confiscated the ship and brought it to the Chennai Port. Upon inspection, it was found that Voyager had an ‘Under Water Exploration Robot Aim’ for its actual needs. The said equipment contains many features that are claimed in a patent granted to the Indian Maritime University (IMU). IMU files an infringement case against the owners of the vessel. White Waterlines approaches you for advice. Draft a note for your client suitably advising them in accordance with the provisions of the Patents Act, 1970?
Part B (60 Marks)
Part B1
For any one of the two specifications (1 and 2) stated below,
I. draft 5 claims
II. draft an abstract (maximum of 150 words) and
III. provide an appropriate title
1 X 30 = 30 marks
Specification No. 1
The invention generally relates to a device for cutting or cracking open nuts or hard fruits of variable size. The invention particularly relates to a device for cutting open coconuts of various shapes and sizes for domestic use and use in temples and in small-scale industries.
Coconuts find extensive use in domestic and industrial applications in various forms such as coconut shavings, coconut water, coconut-milk, coconut oil, desiccated coconut, coir etc. Coconut is also used in foods, cosmetics, personal care products, neutraceuticals, etc. In many countries, coconuts falling from trees are wasted due to high costs involved in plucking and breaking coconuts. Breaking of coconuts is usually done manually. Holding a coconut in one hand and using a heavy metallic object in the other hand to break the coconut tends to damage the nerves and muscles of the operator over a period of time,
A need therefore exists for a device that can be used in homes, temples, hotels, and restaurants. It should be simple, easy to operate and flexible enough to cater to different sizes and types of coconuts, with a capability of being automated.
Description:
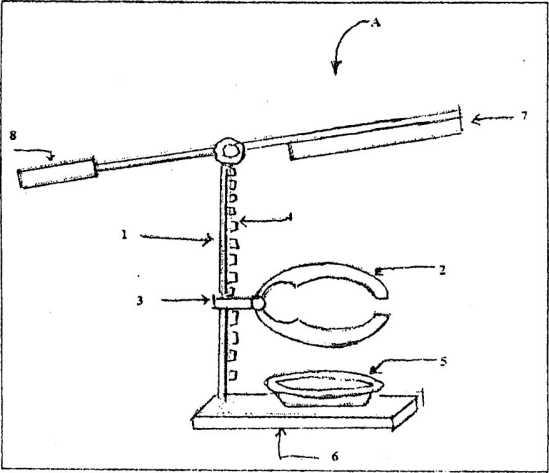
As illustrated in Fig. 1, the coconut breaking device has a vertical stand (1) having grooves and notches (4), a coconut holder (2) having an adjustment knob (3) and a receptacle / container (5) placed over a base (6). The vertical stand (1) is connected to the base (6) at its one end and is connected to a rod, which has a fulcrum at the point where the said rod is connected to the vertical stand (1). This rod has a sharp edged laminar blade/knife (7) at its one end and a handle (8) at its other end. The base (6) can be mounted or attached’rigidly to any flat surface for operation. The grooves and notches (4) on the vertical stand (1) allow appropriate vertical positioning of the coconut holder (2). The receptacle (5) is provided at the base (6) to collect coconut water. The prong like structure of the coconut holder (2) allows the coconut to be grasped firmly and thereby minimizes the risk of accidental slippage.
A coconut is held in between the prongs of the coconut holder (2). Vertical position of the coconut holder (2) is adjusted suitably. An operator moves the handle (8) in the upward direction causing the knife (7) to come into contact with the coconut, placed in the coconut holder (2) thereby slicing the coconut into two pieces. The motion can be as swift as the situation demands, i.e. depending on the outside crust of the‘coconut. The coconut water is collected in the receptacle (5). The handle (8) is made of a material that prevents injury to the hand of the operator e.g. leather, cloth etc.
In an embodiment of the invention, the process can be automated by using electrically operated parts. Any such embodiment will fall within the scope of this invention.
The invention in general relates to substances for removing dirt from leather and/or for coloring the leather. In particular, the invention relates to a shoe polish.
The object of the invention is to provide a shoe polish which may be readily and easily applied to ordinary leather shoes to color them with any desired tint, or to match or harmonize them with the costume of the wearer. A further object of the invention is to provide a shoe polish as mentioned which may be readily removed from the shoes when it is desired to change the color thereof. A further object of the invention is to provide a shoe polish as mentioned which will not be deleterious to the leather but which on the contrary will serve to keep the leather soft and pliable. Other objects will appear hereinafter.
In carrying out the invention, preferably the shoe polish (or dressing) is applied to the leather in the seven positive colors namely: yellow, red, blue, green, brown, black, and white. The user can also compound the desired tints therefrom as needed, without departing from the scope of the invention.
Description:
The composition of the shoe polish is as follows :—
Powdered coloring matter 4 parts by weight
Water, containing a small quantity of salt, sodium carbonate and an egg 8 parts by weight
Lard oil 1 part by weight
Syrup (corn or cane or mixture of both) 4 parts by weight
Mucilage formed of gum arabic and water 8 parts by weightage
4 parts by weight of powdered coloring matter is ground well into 8 parts of a first mixture of salt, sodium carbonate and a well-beaten raw egg in 1 litre of warm water to fonn a second mixture. About 60 grams of salt, 15 grams of sodium carbonate and 60 grams of beaten egg will usually be sufficient. The coloring matter is preferably chrome yellow, chrome red, ultramarine blue, chrome green, burnt umber, bone black, and zinc white. To twelve parts of the said second mixture thus formed is added 1 part of lard oil with stirring after which are added four parts of syrup and eight parts of mucilage. The syrup used is preferably ninety per cent com syrup and ten per cent cane syrup, although either may be used separately or in different proportions; and the mucilage is formed by dissolving 500 grams of gum arabic in 2.5 litres of water resulting in the final composition, which is allowed to stand for one week after which it is ready for use. Egg is added to the water for the color and it also helps to keep the leather soft and adds luster to the polish. The salt preserves the egg and the sodium carbonate neutralizes the acids in the color. The lard oil preserves the leather and keeps it soft and pliable and also prevents the polish from cracking. The syrup and mucilage are added for an adhesive and to give luster to the polish.
In using the polish (or dressing) two or more of the colors may be mixed together to form a dressing of the desired tint. Usually two applications of the dressing will give an even color although sometimes three or more may be preferred. Unless the dressing has remained upon the leather for a great length of time it may be readily washed off to be replaced by another tint.
Part B2
A client meets you and provides technical information regarding his invention. Draft a complete specification, for any one of the following descriptions, for filing in the Indian Patent Office.
While preparing the complete specification, do not redraw the figures. However, you may refer to the figures in the specification as Fig. 1, Fig. 2 and Fig. 3.
1 X 30 = 30 marks
Question 1
This invention is about a down-flow drinking straw that delivers liquid below the water level of a drinking vessel, while eliminating siphoning after an individual has stopped drinking, to reduce the amount of liquid spilled. The invention finds application in hospitals, convalescent homes and private homes for use by bed-ridden individuals.
We are familiar with the use of a straw that is used to sip liquid from a cup or a glass. A problem arrises when the delivery end of the straw is below the fluid level of the glass as a siphon is created due to which even after the person stops sucking the straw, fluid continues to flow out from the straw. This can be undesirable as liquid can spill on the person’s face and clothes. There is a lot of prior art dealing with this problem but no straws with antisiphoning features have been invented yet. Complicated straws with a variety of check valves to avoid back flows are however available. Straws with controlled pumping systems are also available. The present invention overcomes all the drawbacks of the known straws.
Figures 1 provides a schematic diagram of the construction of the straw of the present invention. Figure 2 provides a view of a patient using the straw in a hospital.
Note the straw having a straight supply tube portion connected by an adjustable bend to another straight pickup tube portion with an increased diameter portion below the adjustable bend. Also note the nature of the adjustable bend so that the supply tube portion is above the increased diameter portion when the straw is released. Once the supply tube portion is above the increased diameter portion of the tube, the increased diameter portion provides a volume of liquid to reverse the siphon and pulls the remaining liquid in the supply tube portion back into the glass. The volume of liquid‘in the enlarged diameter portion of the straw exceeds the volume of the entire supply tube portion.
Several variants of the straw are possible. Figure 3 illustrates one such variant.
204
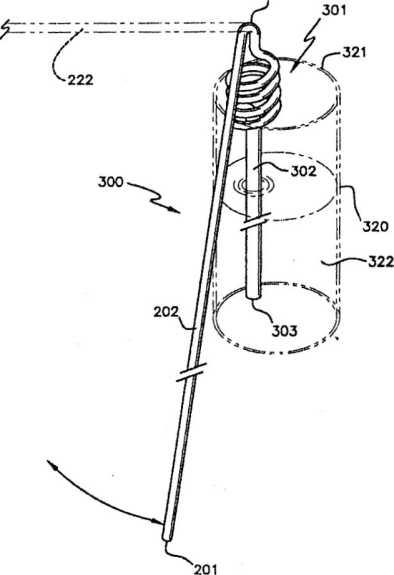
A side view of a glass containing a variant of the anti-spilling down flow drinking straw, having a spiraling pickup tube
In the variant illustrated in figure 3, the straw has a long straight supply tube portion connected by an adjustable bend to another straight pickup tube portion, with an increased diameter portion in the supply tube portion that extends from below the adjustable bend downwardly. In this case, the spiral sections of straw connect the increased diameter portion to the adjustable end. The spiral sections are most useful when a container having a cover with a small opening is used (such as aluminium can). The straw is rotated and the lower spiral sections of the straw are routed through the opening and below the cover. This results in the spiral sections of the straw gripping the cover from below and above thus supporting the straw on the container. By supporting the straw’s pickup tube portion, the spring action of the adjustable bend is better able to lift the supply tube portion to the correct height for draining the straw. The spring action itself can be provided by the spiral sections. The increased diameter portion in this embodiment extends to the bottom of the pickup tube.
The actual material used to make the straw may be any of a number of available plastics, acrylics, polyurethanes, etc., as long as the material provides the spring function of the adjustable bend. Various colours and reservoir shapes may also be envisioned for aesthetic and entertainment purposes.
Question 2
Bilayer tableting technology has become popular in recent times. Bilayer tablets offer several advantages over conventional tablets.
The standard generic dosage forms of Analgesic-Antipyretic drugs have maximum effectiveness only for a few hours, e.g. 4 to 6 hours. Therefore, a patient needs to take such medication as least 2 to 3 times a day, which is undesirable.
It is desirable to offer patient friendly dosage forms that need to be taken only once day and yet ensuring uniform concentration of the drug in the serum for a 12 to 24-hour period. Patient compliance will be maximized when frequent dosing is avoided and relief is available for a longer period.
Formulating oral dosage form of Aceclofenac has remained a challenge in view of its poor or lack of solubility. Aceclofenac being insoluble in water, needs to be combined with solubilizers to improve the bioavailability. The disease conditions where Aceclofenac is used requires immediate relief and effectiveness on a continued uniform level for a prolonged period of time.
By “immediate release core”, it is meant for purposes of the present invention that the tablet core containing the therapeutically active agent(s) meets the disintegration and/or dissolution requirements for immediate release tablets of the particular therapeutically active agent(s) included in the tablet core, as set forth in the USP XXII, 1990 (The United States Pharmacopeia).
By “sustained release”, it is meant for purposes of the present invention that the release of the therapeutically active agent occurs such that blood levels are maintained within a desired therapeutic range over an extended period of time, e.g., at least about 8 and preferably from about 12 to about 24 hours. The “dissolution requirements” and “disintegration requirements” referred to above are conducted using the equipment and tests specified in the USP XXII and conducted pursuant to the individual Official Monographs of USP XXII for the particular therapeutically active agent(s) included in the tablet core.
Prior art discloses
• analgesic composition with uniform bioavailability over a prolonged period.
• sustainable oral dosage forms of aceclofenac.
The invention discloses oral dosage pharmaceutical composition of aceclofenac that provides uniform blood level concentrations over 12 hours to 24 hours a day. A bilayer oral dosage form of the water insoluble drug is formulated with a first layer which offers immediate release and a second layer which offers the drug from a sustained release matrix over 12 hours to 24 hours a day. The first layer for immediate release is formulated to release aceclofenac into the blood stream to initiate and achieve peak level concentrations as desired within 15 minutes.
The immediate release component is prepared by mixing 30% of total Aceclofenac in the composition with Betacyclodextrin along with pharmaceutically acceptable excipients. The sustained release component was prepared by mixing 70% of total Aceclofenac with pharmaceutically acceptable excipients selected from PVP, hydroxyl propyl methyl cellulose (HPMC), carboxy methyl cellulose (CMC), glyceryl monosterate poloxamer and surfactants, based on hydrogenated castor oil (PEG-60, PEG-40).
The Aceclofenac composition has following advantages :
(a) Rapid availability in bloodstream and hence early onset of relief.
(b) Uniform plasma level concentrations of aceclofenac over prolonged period.
(c) Lower frequency of dosage and hence better patient compliances.
The bilayer tablet is prepared using the granules/pellets of immediate layer prepared by solvent/water based dissolution followed by dry granulation process and the sustained release granules/pellets prepared by the non-aqueous wet granulation process and compressing the two grades of granules/pellets using a C-300 or CTX II A Cafrnark Rotary Tablet Press. The bilayer tablet can be prepared either as a tablet in tablet form or as a conventional bilayer tablet using the above equipment.
The tablet prepared by compression is thereafter subjected to standard evaluation procedures such as disintegration, dissolution as well as bioavailability studies to determine that the desired blood level concentrations over a 12 hour or 24 hour sustained release period is met as preset in the objective of the formulation.
Examples
Aceclofenac in the present invention is incorporated in a total quantity of lOOmg for standard SR dosage from and 200mg for Forte dosage form.
Immediate Release layer :
30gms Aceclofenac is dissolved in 50ml solvent selected from acetone or ethyl alcohol or a combination thereof and mixed with Betacyclodextrin (50gms) dissolved in 30ml de-ionized water. The mixture is stirred to evaporation with moderate heating (40 to 50°C) to form a uniform paste comprising aceclofenac-betacyclodextrin complex. The complex so obtained is subjected to dry granulation by precompression followed by granulation or pelletisation and blended with lubricants.
70gms of aceclofenac is dissolved in 50ml of acetone or ethyl alcohol or a combination thereof. 50gms of HPMC and 30gms of Povidone is added followed by 0.5gms of sodium lauryl sulfate along with 50 ml of deionized water.
The resultant solution was spray dried to produce a solid powder using a standard spray dryer. The solid powder is subjected to dry granulation by blending with lubricants by precompression or roll compaction followed by granulation/pelletisation.
The granules of the immediate release layer and the granules of the sustained release layer are directly fed into the tablet compression machine to obtain a standard bilayer tablet or a tablet in tablet dosage form. The tablet is subjected to standard tests such as disintegration, dissolution as well as compliance to the preset bioavailability parameters.
The immediate release layer composition is :
Aceclofenac – 30% of the total aceclofenac in the composition
Betacyclodextrin – 5 to 10% of Aceclofenac in the immediate release layer.
Pharmaceutical excipients for granulation – 2 to 3% of the Aceclofenac in the immediate release layer.
The sustained release layer composition is :
Aceclofenac – 70% of the total aceclofenac in the composition
Hydroxy propyl methyl cellulose – 40 to 50% of Aceclofenac in the sustained release layer.
1-venyl 2-pyrrolidone – 50 to 60% of the Aceclofenac in the sustained release layer.
Pharmaceutical excipients for granulation – 5 to 10% of the total weight of the sustained release layer.
Refer to the official website for the latest question papers : http://www.ipindia.nic.in/Patent-Agent-Examination.htm





